29,000
acres and more serve as a real-life laboratory for plant research
30,100
alumni and counting making a difference
2
Nobel Laureates and 18 National Academies members on the faculty
Undergraduate
Students
Graduate
Students
CNAS Science News

April 18, 2024
Solving antibiotic and pesticide resistance with infectious worms
To study how parasites evolve to break the defenses of their hosts, the National Institutes of Health has granted UC Riverside nematologist Simon “Niels” Groen a $1.9 million Outstanding Investigator Award.

April 17, 2024
CO2 worsens wildfires by helping plants grow
By fueling the growth of plants that become kindling, carbon dioxide is driving an increase in the severity and frequency of wildfires, according to a UC Riverside study.
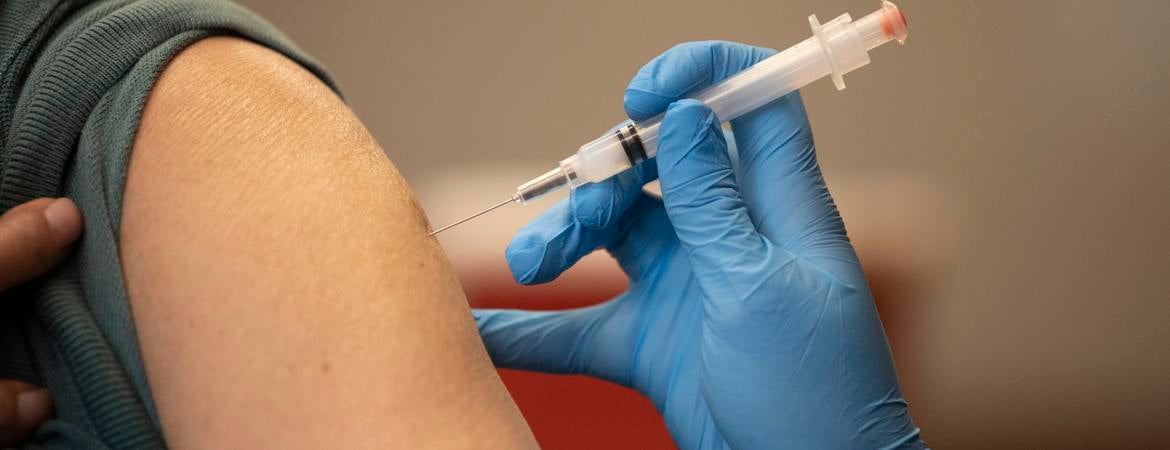
April 15, 2024
Vaccine breakthrough means no more chasing strains
Scientists at UC Riverside have demonstrated a new, RNA-based vaccine strategy that is effective against any strain of a virus and can be used safely even by babies or the immunocompromised.

April 12, 2024
Physicists solve puzzle about ancient galaxy found by Webb telescope
UC Riverside study offers an explanation for dark matter distribution in a massive quiescent galaxy
A Message from Interim Dean Peter Atkinson
On behalf of the faculty, staff, and students from the College of Natural & Agricultural Sciences at UC Riverside, I welcome you to our college and campus.
More College News
CNAS Events
Inside UCR
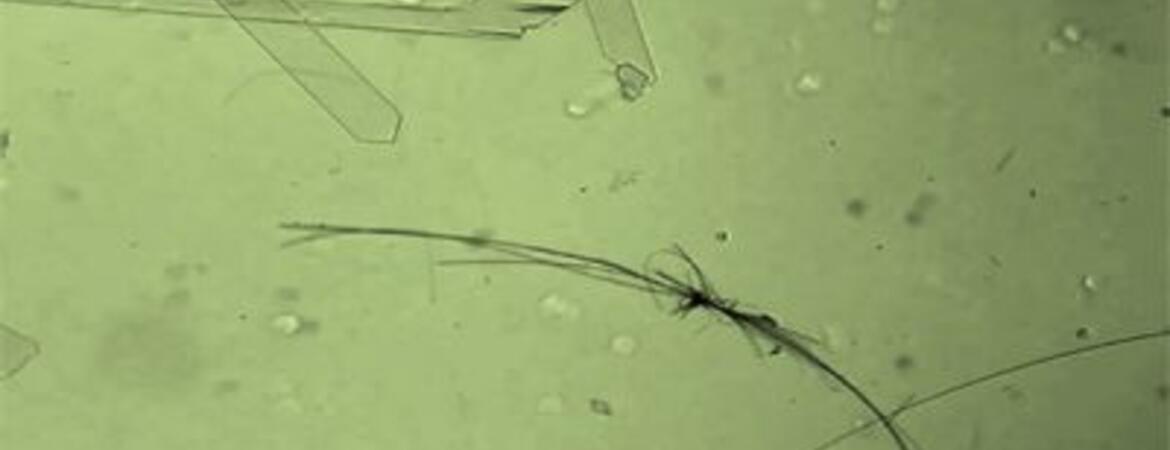
April 11, 2024
Molecular crystal motors move when exposed to light
Potential applications include drug delivery

April 10, 2024
Graduate students recognized for academic excellence and research achievements
Stipends will support their ongoing research

April 09, 2024
Extracurricular: Chasing this year’s spectacular solar eclipse
Eclipse enthusiast Morris Maduro joins millions of people along path of totality

March 13, 2024
Undergraduate wins UC Graduate Deans’ Leadership and Research Award
Budding neuroscientist Erik Hakopian provided academic mentorship to UCR students
































